 |
Back to Parasitoids Table of Contents

Tamarixia radiata Waterston
[Hymenoptera: Eulophidae],
an ectoparasitoid of Diaphorina citri
Kuwayama [Hemiptera: Psyllidae]
by Jawwad A. Qureshi and Philip A. Stansly University of Florida/IFAS Department of Entomology and Nematology Southwest Florida Research and Education Center, Immokalee, FL 34142
Tamarixia radiata is a species specific ectoparasitoid of the Asian citrus psyllid (ACP) Diaphorina citri. Diaphorina citri is an economically important pest of citrus in many citrus growing regions of the world (Halbert and Manjunath, 2004). The psyllid vectors the phloem limited gram-negative bacterium Candidatus Liberibacter asiaticus, one of the causal organisms of the devastating citrus disease “huanglongbing” (HLB) or citrus greening disease. HLB is responsible for the decline of most trees seen in disease affected regions of the world (Roistacher 1996; Garnier et al. 2000; Halbert and Manjunath, 2004; Bové 2006).
In the United States, ACP and HLB were first identified from Florida in 1998 and 2005, respectively (Halbert, 1998, 2005). The psyllid is now well established in citrus producing regions of the state (Halbert and Manjunath, 2004; Qureshi et al., 2009) and is also present in Texas, Louisiana, Mississippi, Georgia, South Carolina, Hawaii and southern California (French et al., 2001; FDQO-CG-ACP, 2008). The disease now occurs throughout Florida with highest incidence in the east coast and southwest regions and threatens a citrus industry valued at $1.3 billion in direct sales alone (Anonymous 2008; FDACS-DPI 2008). Other than plant movement by people, D. citri is the primary cause of the spread of the HLB disease (Halbert and Manjunath, 2004); therefore, effective and sustainable pest and disease management strategies are required to reduce spread.
Biological control is an essential component of integrated pest and disease management. Tamarixia radiata is the most common the two well known primary parasitoids of D. citri and thus a strong candidate for classical biological control of this pest. Tamarixia radiata was first imported to Florida from Taiwan and south Vietnam and limited numbers were released soon after psyllid was discovered in the state (Hoy et al., 1999). Evaluation of parasitism of D. citri by T. radiata at 28 citrus groves across 16 counties in 2006-2007 showed that parasitism rates were generally low, rarely exceeding 20% (Qureshi et al. 2009) in contrast to other areas such as Caribbean and Réunion Islands (Pluke et al, 2008, Étienne and Aubert, 1980). Therefore, we began importing new parasitoids in 2008 from China, Pakistan and north Vietnam and established colonies of both previously established and new parasitoids which are being released and evaluated in Florida (Qureshi and Stansly, unpublished). Other programs of release and evaluation are being conducted in Texas, Mexico and Costa Rica (D. Flores, H. Arreondo, and D. Brown, personal communications).

Origin
Tamarixia (=Tetrasticus) radiata was first described from northwestern India (Punjab) which is now part of Pakistan (Waterston 1922).

Appearance
Adults are small wasps that range from 0.92 to 1.04 mm in length (Onagbola et al. 2009). Males are slightly smaller than females both in body length and wing span. Antennae are geniculate and can be used to separate males and females. Male antennae are 1.5 times longer than those of female (Onagbola et al. 2009) and contain long and slightly curved hairs or setae compared to female antennae with short setae. Head and thorax are shiny black in both genders. The posterior dorsal and lateral sections of the gaster are black while the venter and a patch of the anterior dorsal gaster are clear to yellow.

Habitat
Tamarixia radiata was recovered throughout the year from fourth and fifth instar nymphs of D. citri collected from 26 citrus groves in the central, southwest, and eastern coastal regions of Florida (Qureshi et al. 2009). However, parasitism rates were variable and averaged <20% during spring and summer over all locations. Incidence of parasitism increased during fall at some locations, averaging 39% in September and 56% in November in the central and southwest regions, respectively (Qureshi et al. 2009). Overall, parasitism rates were higher in the southwest region (21%) followed by the central (10%) and eastern coastal (8-12%) regions. Fall is relatively cool and dry compared to hot and humid spring and summer and temperatures are relatively higher in the south than in the north. Parasitism rates from Puerto Rico were much higher and ranged from 79 to 88% between January and April. The relatively more extreme climate of Florida compared with Puerto Rico may reduce parasitoid populations. However, other factors such as high levels of predation, use of insecticides, poor overwintering, or inherent biological characteristics of the parasitoid may be responsible for low parasitism rates in Florida (Michaud 2004, Qureshi and Stansly 2009).

Pests Attacked
Diaphorina citri is the only known host of T. radiata

Life Cycle
The female usually deposits one and very rarely two eggs on the ventral side of the D. citri nymph, mostly in the groove between thorax and abdomen and close to the point of attachment of hind coxae. The neonate larva sucks fluid from the site of attachment and later on moves to neighboring regions as it consumes more body contents and develops underneath the nymph. The parasitized nymph remain indistinguishable from unparasitized nymph for couple of days until almost all body contents of the parasitized nymph are consumed and only wing buds and dorsal covering are left. At that point, the larva is mature and attaches itself to the leaf or shoot surface with a network of fine anchoring silken threads which are later visible around the margins of the mummified nymph. Pupation takes place underneath the mummified nymph and the adult emerges by chewing a circular exit hole in the integument of the thoracic region. Development rate varies with temperature. It takes about 12 days to go from egg to adult at 25 ºC and 14L:10D. A single female can lay up to 300 eggs at 25-30 ºC (Chu and Chien, 1991; Étienne et al. 2001).
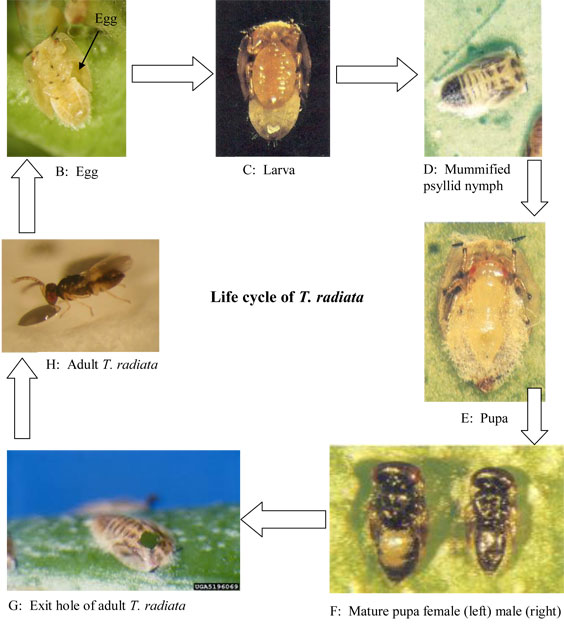
PHOTOS: B and H: A. Urbaneja, CPVB IVIAM, Spain
C, D, E, and F: C. C. Chien, S. C Chiu and S. C. Ku. TARI, Taiwan
G: University of Georgia

Relative Effectiveness and Conservation
Females of T. radiata host feed on the younger instars of D. citri and prefer to oviposit underneath the later instars, particularly fifth instar (Chu and Chien, 1991). Through combined behaviors of host feeding and oviposition, a single female is capable of destroying 500 D. citri nymphs during her lifetime (Chien 1995). Tamarixia radiata killed 90% of the presented nymphs through parasitism and host feeding in laboratory experiments (Aubert 1991, Skelley and Hoy, 2004). Tamarixia radiata was released in Réunion, Taiwan, and Guadaloup (Aubert and Quilici 1984, Chien 1995), and was credited with reducing the populations of D. citri sufficiently in Réunion to mitigate the impact of citrus greening disease (Étienne and Aubert, 1980; Chien and Chu, 1996; Étienne et al. 2001). The parasitoid was also detected in Brazil, and Puerto Rico, where no known releases were made (Torres et al. 2006, Pluke et al. 2008). Incidence of parasitism at Isabela, Puerto Rico ranged from 79 to 88% between January and April. High rates of parasitism in spring were followed by continuously reduced psyllid populations during summer. Parasitism rates generally exceeded 50% and averaged 70% at Isabela, but were more variable and averaged 38.5% at Corozál and Gurabo when psyllid populations were lower and more variable.
Tamarixia radiata was imported from Taiwan and south Vietnam, and was released in Florida in 1999 (Hoy et al. 1999, Hoy and Nguyen, 2001). Approximately 37,000 adults of a mixed colony from the two origins were released in Florida citrus between 1999 and 2001 (Skelley and Hoy 2004). In a study conducted during 2006-2007, T. radiata was recovered from fourth and fifth-instar psyllid nymphs at 26 of the 28 groves distributed across the state (Qureshi et al. 2009). However, parasitism rates were much lower compared to other regions such as Réunion, Guadaloupe and Puerto Rico.
Attempts are being made to enhance biological control of D. citri by augmenting populations of T. radiata with mass-reared T. radiata of the already established strain originally imported from Taiwan and south Vietnam and new colonies that we imported from Pakistan, China and north Vietnam. The colony of already introduced strain was established at UF-Southwest Florida Research and Education Center, Immokalee, FL, and colonies from Pakistan, China and north Vietnam populations were established at Division of Plant Industry, Gainesville, FL. Releases of the already established strain and the new strains began in Spring and Fall of 2009, respectively. These parasitoids are being released in both organic and conventional citrus groves and seem to increase the parasitism rates compared to control groves where no releases are being made. For example, in Fall 2009 parasitism averaged 50%

Conservation
We have demonstrated that when using foliar insecticides, more psyllid control is achieved by targeting adults either during the dormant winter period when citrus trees are not flushing or between the flush cycles during the growing season (Qureshi and Stansly 2010, Stansly et al. 2009b). Such an approach conserves many psyllid natural enemies
that are largely absent from citrus groves during the dormant season but migrate in when trees are producing new growth which is essential for reproduction of psyllids and other prey such as aphids. However, T. radiata must also overwinter in citrus groves and is likely to be impacted by dormant sprays. Therefore, it is important to repopulate the groves in spring when psyllid nymphs are available to be parasitized. Psyllid control during the growing season should be based on scouting to further reduce the use of insecticides and conserve beneficials (Stansly et al. 2009a).

Pesticide Susceptibility
Sixteen pesticides including two fungicides were evaluated for toxicity to adult T. radiata (Hall and Nguyen 2010). Percentage mortality data were evaluated to generally assess IPM compatibility of the pesticides with adult parasitoids. Carbaryl, chlorpyrifos, and fenpropathrin were found to be least compatible with (most toxic to) adult T. radiata based on the toxicity of direct sprays and potential long residual life on leaves. Although highly toxic to the parasitoid as direct sprays or freshly dried residues, each of the following was more compatible with T. radiata because their residual toxicity was either low at one to three days after application or relatively non-persistent: abamectin, chenopodium oil, fenpyroximate, and spirotetramat. Depending on environmental conditions, imidacloprid (foliar-applied), phosmet, pyridaben, sulfur and 435 spray oil might also be somewhat more compatible for the same reasons. The pesticides that consistently appeared to be most compatible with T. radiata were fosetyl-aluminum, copper hydroxide, diflubenzuron, and kaolin clay (Surround WP).

Commercial Availability
Tamarixia radiata is not yet available commercially.

References
Anonymous, 2008. Citrus Summary 2006-2007. Florida Agricultural Statistics Service, Florida. Florida Department of Agriculture and Consumer Services.
Aubert, B. 1991. Biological control of Diaphorina citri, a vector of citrus greening disease, pp. 118-119. In Proceedings, Biological Control of Plant Diseases and Virus Vectors Conference, 17-21 September 1990, Tsukuba, Japan. Food and Fertilizer Technology Center for the Asian and Pacific Region, Taipei, Taiwan.
Aubert, B., and S. Quilici. 1984. Biological control of the African and Asian citrus psyllids (Homoptera: Psylloidea), through eulophid and encyrtid parasites (Hymenoptera: Chalcidoidea) in Reunion Island, pp. 100-108. In S. M. Garnsey, L. W. Timmer, and J. A. Dodds [eds.], Proceedings, 9th Conference of the International Organization of Citrus Virologists (IOCV), 9-13 Nov. 1983, Argentina. University of California, Riverside, CA.
Bove´, J. M. 2006. Huanglongbing: a destructive, newly emerging, century-old disease of citrus. J. Plant Pathol. 88: 7-37.
Chien, C. C., and Y. I. Chu. 1996. Biological control of citrus psyllid, Diaphorina citri in Taiwan. pp. 93-105. In Biological Pest Control in Systems of Integrated Pest Management, October 1993. Food and Fertilizer Technology Center for the Asian and Pacific Region, Taipei, Taiwan.
Chu, Y. I., and C. C. Chien. 1991. Utilization of natural enemies to control of psyllid vectors transmitting citrus greening, pp. 135-145. In K. Kiritani, H. J. Su, and Y. I. Chu [eds.], Proceedings, Integrated control of plant virus diseases, 9-14 April 1990. Food and Fertilizer Technology Center for the Asian and Pacific Region, Taichung, Taiwan.
Chien, C. C. 1995. The role of parasitoids in the pest management of citrus psyllid. pp. 245-261. In Proceedings, Symposium: Research and Development of Citrus in Taiwan, Taichung, Taiwan.
Étienne, J., and B. Aubert. 1980. Biological control of psyllid vectors of greening disease on Reunion Island, pp. 118-121. In Proceedings, 8th Conference of the International Organization of Citrus Virologists, 13-31 May 1979, Sidney, Australia. University of California Press, Riverside,
CA.
Étienne, J., S. Quilici, D. Marival, and A. Franck. 2001. Biological control of Diaphorina citri (Hemiptera: Psyllidae) in Guadeloupe by imported Tamarixia radiata (Hymenoptera: Eulophidae). Fruits 56: 307-315.
[FDACS-DPI] Florida Department of Agriculture and Consumer Services, Division of Plant Industries. 2008. Huanglongbing (HLB)/Citrus Greening Disease. (http://www.doacs.state.fl.us/pi/chrp/greening/citrusgreening.php).
(FDQO-CG-ACP) Federal Domestic Quarantine Order, 2008. Citrus greening and Asian citrus psyllid.
French, J. V., C. J. Kahlke, and J. V. Da Graca. 2001. First record of the Asian citrus psylla, Diaphorina citri Kuwayama (Homoptera: Psyllidae), in Texas. Subtrop. Plant Sci. 53: 14-15.
Garnier, M., S. Jagoueix-Eveillard, H. F. Cornje, P. R. Le Roux, and J. M. Bove´. 2000. Genomic characterization of a Liberibacter present in an ornamental rutaceous tree, Calodendrum capense, in the Western Cape province of South Africa. Proposal of „Candidatus Liberibacter africanus subsp. Capensis?. Int. J. Syst. Evol. Microbiol. 50: 2119-2125.
Halbert, S. E. 1998. Entomology section. Triology (May-June) 37: 6-7.
Halbert, S. E. 2005. Pest alert: citrus greening/Huanglongbing. Florida Department of Agriculture and Consumer Services. Division of Plant Industry.
Halbert, S. E., and K. L. Manjunath. 2004. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Fla. Entomol. 87: 330-353.
Hoy, M. A., R. Nguyen, and A. Jeyaprakash. 1999. Classical biological control of the Asian citrus psylla-release of Tamarixia radiata. Citrus Indust. 80: 20-22.
Hoy, M. A., and R. Nguyen. 2001. Classical biological control of Asian citrus psylla. Citrus Indust. 81: 48-50.
Michaud, J.P., 2004. Natural mortality of Asian citrus psyllid (Homoptera: Psyllidae in central Florida. Biological Control 29, 260–269.
Onagbola E. O., D. R. Boina, S. L. Hermann, and L. L. Stelinski. 2009. Antennal sensilla of Tamarixia radiata (Hymenoptera: Eulophidae), a parasitoid of Diaphorina citri (Hemiptera: Psyllidae). Ann Entomol Soc Am 102:523–531
Pluke, R.W.H., J. A. Qureshi, and P. A. Stansly. 2008. Citrus flushing patterns, Diaphorina citri (Homoptera: Psyllidae) populations and parasitism by Tamarixia radiata (Hymenoptera: Eulophidae) in Puerto Rico. Fla. Entomol. 91: 36-42.
Qureshi, J.A., and P. A. Stansly. 2009. Exclusion techniques reveal significant biotic mortality suffered by Asian citrus psyllid Diaphorina citri (Hemiptera:Psyllidae) populations in Florida citrus. Biol. Contr. 50, 129-136.
Qureshi, J.A., M. E. Rogers, D. G. Hall, and P. A. Stansly. 2009. Incidence of invasive Diaphorina citri (Hemiptera:Psyllidae) and its introduced parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) in Florida citrus. J. Econ. Entomol.102, 247-256.
Qureshi, J.A., and P. A. Stansly. 2010. Dormant season foliar sprays of broad-spectrum insecticides: An effective component of integrated management for Diaphorina citri (Hemiptera:Psyllidae) in citrus orchards. Crop. Prot. 29, 860-866.
Roistacher, C. N. 1996. The economics of living with citrus diseases: Huanglongbing (greening) in Thailand, pp. 279-285. In J. V. DA Grac¸ a, P. Moreno, and R. K. Yokomi [eds.], Proceedings, 13th Conference of the International Organization of Citrus Virologists (IOCV), 17-23 Nov.
Skelley, L. H., and M. A. Hoy. 2004. A synchronous rearing method for the Asian citrus psyllid and its parasitoids in quarantine. Biol. Control 29: 14-23.
Stansly,P.A., J. A. Qureshi, and H. A. Arevalo. 2009a. Why, when and how to monitor and manage Asian citrus psyllid. Citrus Industry. 90, 24-26.
Stansly,P.A., H. A., Arevalo, M. Zekri, and R. Hamel. 2009b.Cooperative dormant spray
program against Asian citrus psyllid in SW Florida. Citrus Ind. 90,14-15.
Torres, M. L., D. E. Nava, S. Gravena, V. A. Costa, and J.R.P. Parra. 2006. Registro de Tamarixia radiata (Waterston) (Hymenoptera: Eulophidae) em Diaphorina citri Kuwayama (Hemiptera: Psyllidae) em Sa˜o Paulo, Brasil. Rev. de Agricultura, Piracicaba-SP 81: 112-117.
Waterston, J. 1922. On the chalcidoid parasites of psyllids (Hemiptera, Homoptera). Bull. Entomol. Res. 13: 41-58.

Back to Parasitoids Table of Contents

|
 |
T. radiata female (A)
PHOTO: A. Urbaneja, CPVB IVIAM, Spain
|
|
